Advances in detection, prevention and treatment of heart failure in type 2 diabetes: part I
ALICE C COWLEY, ABHISHEK DATTANI, EMER M BRADY, GERRY P MCCANN, GAURAV S GULSIN
Department of Cardiovascular Science, University of Leicester and the NIHR Biomedical Research Centre, Glenfield Hospital, Leicester, UK
Address for correspondence: Dr Gaurav S Gulsin
Department of Cardiovascular Science, University of Leicester and the NIHR Biomedical Research Centre, Glenfield Hospital, Groby Road, Leicester, LE3 9QP, UK
E-mail: gg149@leicester.ac.uk
Abstract
Management of heart failure (HF) in diabetes has previously centred on aggressive control of cardiovascular risk factors. The benefits of this approach are modest. In the past decade, however, there have been numerous advances in our understanding of HF prevention, detection and treatment which are particularly relevant to people with type 2 diabetes. This review is the first of two that summarise these advances, with Part I focusing on HF prevention and detection.
Br J Diabetes 2024;24(1):16-23
https://doi.org/10.15277/bjd.2024.441
Key words: type 2 diabetes, heart failure, heart failure prevention
Introduction
The 2017-18 UK National Diabetes Audit identified heart failure (HF) as the commonest and deadliest major complication of diabetes; even if all major cardiovascular (CV) risk factors are optimised, people with diabetes still have twice the risk of developing HF.1-5 Among the plethora of multisystem diabetes- related complications, HF remains the leading cause of hospitalisation.2 Moreover, for a person with type 2 diabetes (T2DM), rates of death in the year following HF admission are 2-5 times higher than that of the general population.2 The major contribution of HF to morbidity and mortality in people with diabetes was highlighted by the American Diabetes Association in a recent consensus report recognising HF as “an underappreciated complication of diabetes”.6
Classification of HF
Several methods of classifying patients with HF have been proposed, including: categorisation according to symptom severity,7 ejection fraction (EF),8 or a combination of the presence of co-morbidities, symptoms, elevated blood biomarkers and structural heart alterations.9 Most widely applicable is the combined approach described by the American Heart Association, American College of Cardiology and the Heart Failure Society of America in 2001,10 which depicts a four-stage HF continuum:9 Stage A “at risk” includes all people with T2DM given their markedly increased risk; Stage B are those with asymptomatic cardiac structural and/or functional alterations;11 Stage C represents people with signs and/or symptoms of HF irrespective of left ventricular (LV) EF; and Stage D are those with limiting symptoms and recurrent hospitalisations despite maximally tolerated treatment (Figure 1).9 Importantly, there is a decline in survival rates from Stages A to D.12
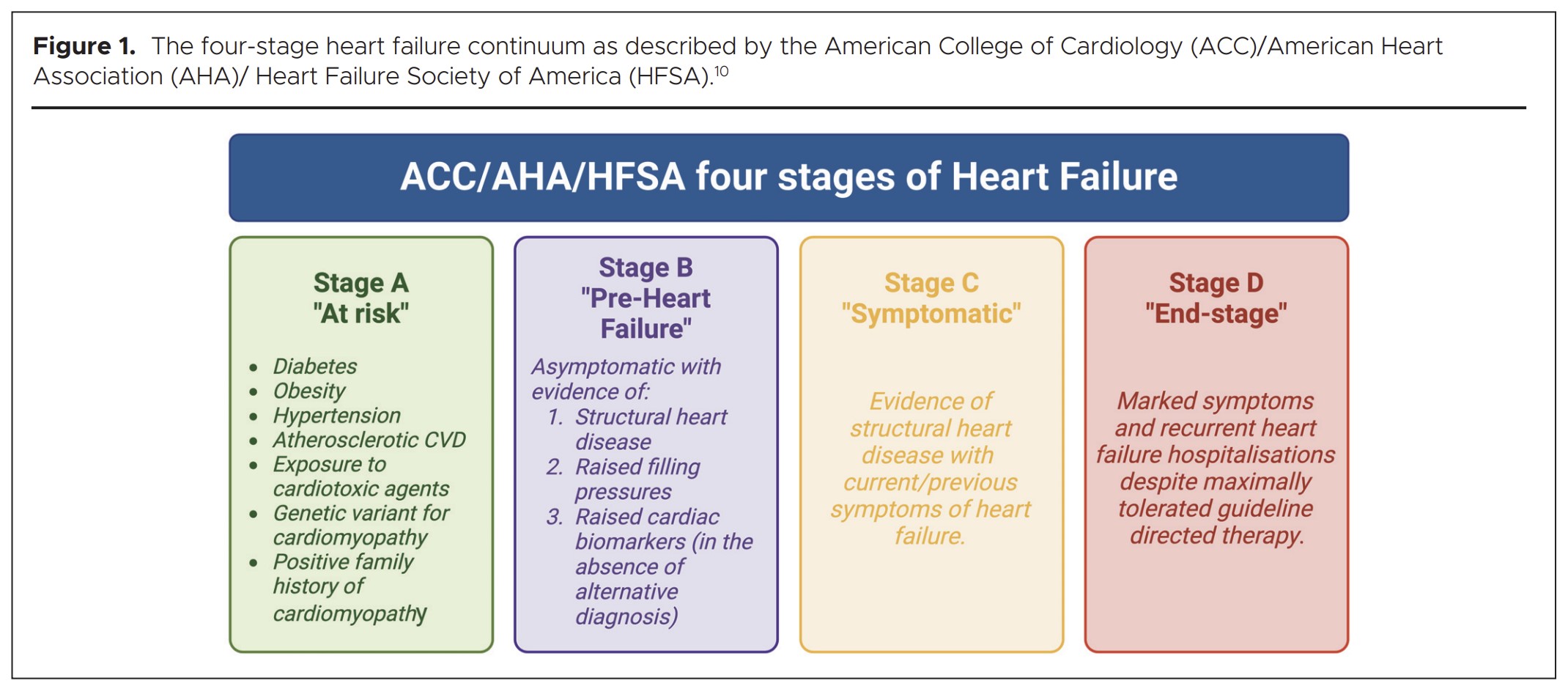
Stage A – prevention of heart failure in at-risk subjects
Early studies that focused primarily on the impact of strict glycaemic and blood pressure (BP) control suggested that the benefits on HF risk reduction were modest.13-15 The Swedish national diabetes registry has shown that good control of diabetes and CV risk factors negates the increased risk of atherosclerotic disease, but there remains a significant excess risk of hospitalisation for HF.1 While maintenance of one or more risk factors within target ranges was associated with a stepwise reduction in the risk of HF hospitalisation, this was not to the same extent as the reduction in other CV events.1 Nevertheless, addressing modifiable risk factors still has an important role to play in treatment for people with T2DM.
Smoking cessation
Multiple studies have reported an increased risk of incident HF in smokers,16-18 and a dose-response relationship between smoking exposure, brain natriuretic peptide (BNP) levels and incident HF hospitalisation.16 Longitudinal observational data have shown that smoking cessation can reduce the downstream risk of HF, with former smokers (who have ceased smoking >15 years previously) having a similar risk of HF incidence and all-cause mortality to people who have never smoked.19 The benefits of smoking cessation are attenuated in people with a history of heavy smoking (>32 pack years), who have a higher risk of HF and all-cause mortality compared to never smokers, but remain at a lower risk of death when compared to current smokers.19 The emergence of e-cigarettes as “safe alternatives” to tobacco smoking has been the subject of much debate. However, a growing evidence base indicates that e-cigarettes are likely to have multiple deleterious effects on general and CV health, particularly increasing BP and atherogenesis.20 In a recent large-scale US observational registry study (n=175,667, average age 52 years, 61% female, median follow-up duration 45 months) specifically evaluating risk of HF, e-cigarette use was associated with a 19% excess risk of incident HF and particularly heart failure with preserved ejection fraction (HFpEF), even after adjustment for age, sex and traditional CV risk factors.21 Although this study included people with and without T2DM, it is reasonable to conclude that e-cigarettes should not be considered an alternative to tobacco smoking in people who have never smoked and may not be a harm-reduction alternative to tobacco smoking in current smokers. Further work is needed to elucidate the precise mechanisms by which e-cigarettes potentiate CV risk and HF in all groups, including people with T2DM.
Physical activity
Large observational prospective cohort studies have shown that greater physical activity is associated with significantly lower CV and overall mortality in men and women with diabetes.22,23 Similarly, higher levels of physical activity and fitness have been shown to reduce the risk of HF.24-29 This was demonstrated specifically in people with T2DM in a subgroup analysis of the ARIC study, which confirmed that increased physical activity reduces the risk of HF in T2DM.30 Exercise training has also consistently been found to lower haemoglobin A1c (HbA1c) by 0.6-0.7%, with greater effects reported with higher exposure to exercise, and benefits observed irrespective of weight loss.31,32 In health, guidance suggests a minimum of 150 minutes/ week moderate-to-vigorous intensity aerobic exercise for adults, supplemented with two to three resistance sessions/week.1,2,6,7,33,34 The European Society of Cardiology (ESC) expand on this to suggest that structured exercise programmes should be introduced for people with T2DM and established CV disease, and that exercise interventions should be adapted in patients with T2DM-associated complications, including frailty.34 Similarly, the World Health Organization states that those with chronic disease (e.g. T2DM) may increase moderate-intensity aerobic activity to >300 minutes (or >150 minutes of vigorous-intensity activity) per week.1 Although individuals are encouraged to work towards these recommendations, physiological benefits can be achieved at levels below and above these thresholds, depending on CV and metabolic training goals; therefore, individualised exercise training programmes are advocated in people with T2DM.35
Weight loss
The Framingham Heart Study reported that for every unit increase in body mass index (BMI) there is an associated 5-7% increased risk of HF.36 Furthermore, there was evidence of a sex-specific interaction between BMI and HF risk, with a linear association observed in men and a J-shaped association in women.37
The metabolic benefits of weight loss in T2DM, including remission of diabetes, are now well established.38 Observational studies have shown that dietary weight loss in people with diabetes is associated with improvement in a range of associated CV risk factors (e.g. low-density lipoprotein [LDL] cholesterol, BP and glycaemia), with the magnitude of weight loss generally being associated with greater risk factor reduction.39 Historically, this was observed to be associated with lower all-cause and CV mortality,40 although the impact on risk of incident HF was not specifically examined. The randomised controlled Look AHEAD trial compared intensive lifestyle intervention (a combination of a dietary weight loss and exercise programme) versus a diabetes support programme.41 In 5,145 overweight/obese people with T2DM (mean age 58.7 years, diabetes duration five years, BMI 36 kg/m2) there was no difference in the rate of CV events or HF development over a median follow-up duration of 9.6 years,41 despite greater weight loss (8.6% vs. 0.7% at one year; 6.0% vs. 3.5% at study end), increased fitness and improved glycaemic control in the intensive lifestyle intervention arm.42 Intensive medical management of CV risk factors was delivered to both intervention groups, which may account for the limited treatment effect. In participants who achieved >10% weight loss, a secondary analysis of the trial cohort found 24% reduced incidence of CV disease (although the composite primary and secondary outcomes were largely comprised of atherosclerotic CV events), highlighting that more modest weight loss may not be sufficient to mitigate CV disease development.43 This is supported by a further subgroup analysis of the same trial, which showed that reducing fat mass and waist circumference (but not lean mass) in the intensive lifestyle intervention arm was associated with lower risk of incident HF.44 At one-year follow up, a 10% reduction in fat mass was associated with 22% lower risk of HFpEF and 24% lower risk of heart failure with reduced ejection fraction HFrEF.44 A recent meta-analysis of >220,000 people with T2DM confirms that concomitant weight loss and reductions in HbA1c are effective at lowering risk of CV disease (including HF) and death.45
For overweight/obese people with T2DM who are unable to achieve weight loss with lifestyle modification, newer pharmacotherapies and bariatric surgery should be explored.
Pharmacologic weight loss therapies
Advances in our understanding of gut hormones and the existence of bidirectional gut-brain interactions in regulating appetite, gastric emptying and pancreatic hormone secretion have led to the development of novel and effective anti-obesity medications, with positive safety profiles. Glucagon-like peptide- 1 receptor agonists (GLP-1 RA) and therapies in combination with other incretin hormones have emerged. Using these agents, body weight reductions between 10-25% have been demonstrated in phase 3 randomised placebo-controlled trials in people with obesity, with or without T2DM, with associated improvements in cardiometabolic risk factors.46-51 The first CV outcomes trial of a long-acting GLP-1 RA (semaglutide 2.4 mg/ week) in overweight/obese adults was published this year,52 but participants with diabetes were excluded and the study cohort comprised only those with established CV disease (which included a combination of participants with stages A and B HF). Nevertheless, the potential beneficial secondary CV effects are the subject of intense interest.
Lipid-lowering therapy
The therapeutic benefits of cholesterol management and statin therapy in preventing atherosclerotic CV disease are well established.53-56 Current guidance recommends the use of statins in people with T2DM based on CV risk,34,57 although there is limited evidence with regard to the impact of lipid-lowering therapy on HF outcomes. Large-scale, randomised, placebo- controlled trials have reported lower incidence of congestive HF with statin use.56,58 Furthermore, a meta-analysis of treatment with statins in HF reported improved all-cause mortality, CV mortality and CV hospitalisation in HFpEF.59 Newer non-statin lipid-lowering therapies such as proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors are now recommended for secondary prevention of atherosclerotic CV disease in certain patients not meeting non-HDL cholesterol targets,60,61 but their role in primary prevention of CV disease is less clear due to lack of available trial data. There is also debate regarding the potential for harm associated with PCSK9i use in patients with a history of HF, possibly mediated via unwanted effects on cardiac metabolism.62,63 As yet there is insufficient evidence to advocate non-statin lipid-lowering therapies to mediate HF risk in T2DM, especially in those with no prior history of CV disease. While the benefits of lipid-lowering therapy may extend beyond reduction of atherosclerotic CV events, given that coronary artery disease is a leading risk factor for HF there is sufficient evidence to warrant strict adherence to existing lipid-lowering therapy guidelines.64
Glycaemic control
Large-scale observational studies have found that worsening glycaemic control is associated with increased risk of incident HF development, diagnosis of HF at a younger age and higher rates of hospitalisation for HF.65,66 Surprisingly, the majority of previous large, multicentre randomised controlled trials did not demonstrate an improvement in macrovascular outcomes with tight blood glucose control.67-70 Meta-analysis data have revealed no observed benefit of intensive glycaemic control on HF-related outcomes,14 and just a modest reduction in major adverse CV events, primarily driven by a reduction in myocardial infarction, with no overall benefit on all-cause mortality or CV death.72 Given that the excess risk of HF is mostly apparent when HbA1c >7.0%,66 all major national and international guidelines advise aiming for a serum HbA1c <7.0% and avoidance of intensive glucose control.72-75
Since the publication of the EMPA REG OUTCOME trial, and the CV outcomes trials that followed,76 management of hyperglycaemia for CV risk and HF prevention in T2DM has become more nuanced. There is now robust evidence to show that sodium glucose co-transporter 2 inhibitors (SGLT2i) give lower rates of HF hospitalisation in people with T2DM with or at high risk of CV disease.76-79 In the recent ESC guidelines for the management of CV disease in diabetes, SGLT2i are recommended as first-line agents in the vast majority of people with T2DM,34 highlighting their centrality in the management of diabetes. Similarly, multiple randomised trials have shown lower rates of CV events using GLP-1 RA in people with T2DM, with or at high risk of CV disease.50,51,80-82 As with SGLT2i, guidelines recommend GLP1- RA as a preferred medication for people with T2DM.34 The effect of GLP-1 RA on HF events specifically is less clear, although recent meta-analysis suggested reduced risk of incident HF in people with T2DM without pre-existing HF at baseline.83
Blood pressure control
Cardiac remodelling in hypertension may lead to permanent myocardial ischaemia, independent of epicardial obstruction.84 Observational studies have reported the well-known link between hypertension and future risk of heart failure.36,85-87 The ACCORD randomised controlled trial in people with T2DM found no difference in mortality or HF hospitalisation with intensive BP management,15 and a significant increase in serious adverse events has been reported with intensive therapy.15,88 Current guidance recommends a tailored approach to patients, giving particular consideration to frailer individuals, who may benefit from a higher BP target.34,75,89 Lastly, non-adherence to antihypertensives remains a major challenge to maintaining adequate BP control and is common in people with T2DM, especially in younger age groups who have the highest lifetime risk of HF; more than 80% reportedly had low adherence in one study.90 A key risk factor for non-adherence particularly relevant to people with T2DM is polypharmacy,91 and suboptimal BP control in people taking multiple antihypertensives should alert clinicians to the possibility of medication non-adherence. Whilst there are several available methods for identifying non- adherence, liquid chromatography-tandem mass spectrometry techniques are now available for detection of antihypertensive medications in urine or other body fluids and should be considered in patients presenting with persistent uncontrolled BP.92
Stage B – early detection of subclinical cardiac dysfunction
One third of people with T2DM have asymptomatic cardiac structural and/or functional alterations that precede HF symptomatology.11,93 Stage B HF is a dynamic phenomenon, with potential for both progression to overt symptomatic HF and reversal to Stage A. In a meta-analysis of 11 cohort studies comprising 25,369 subjects with an average ~8 years follow-up, rates of progression from Stage B HF to symptomatic HF were almost five-fold higher for systolic dysfunction and 1.7 higher for diastolic dysfunction than in those without stage B HF.94 Diabetes was a major predictor of HF progression, along with age, sex, BP and BMI.94 Conversely, a subset of the Olmsted County Heart Function Study, 8% of whom had diabetes, identified regression of Stage B HF to stage A HF or ‘normal’ in 39% of subjects who underwent repeat echo after four years.95 Individuals who regressed had lower troponin and natriuretic peptide levels, higher LV ejection fractions and lower indexed left atrial volumes.95
Given that Stage B HF is potentially reversible, early diagnosis is desirable. Current recommendations for diagnosis of Stage B HF are based solely on transthoracic echocardiography or blood biomarker criteria (Table 1). These will not, therefore, routinely be available in asymptomatic individuals, nor are the echocardiographic parameters always detailed or easily accessible in standard clinical reports. Numerous additional early deleterious effects on cardiac structure and function have been reported in T2DM, including smaller cardiac chamber volumes, impaired myocardial energy utilisation, coronary microvascular dysfunction, myocardial steatosis, focal and diffuse fibrosis, and dysregulated myocardial calcium handling,96,97 that are not included in the current classification of Stage B HF or detectable with echocardiography.11 This will undoubtedly lead to under- estimation of the prevalence of Stage B HF and missed opportunities for treatment. Acknowledging that routine screening for Stage B HF is not currently possible, detection should be opportunistic and include early investigation whenever possible.
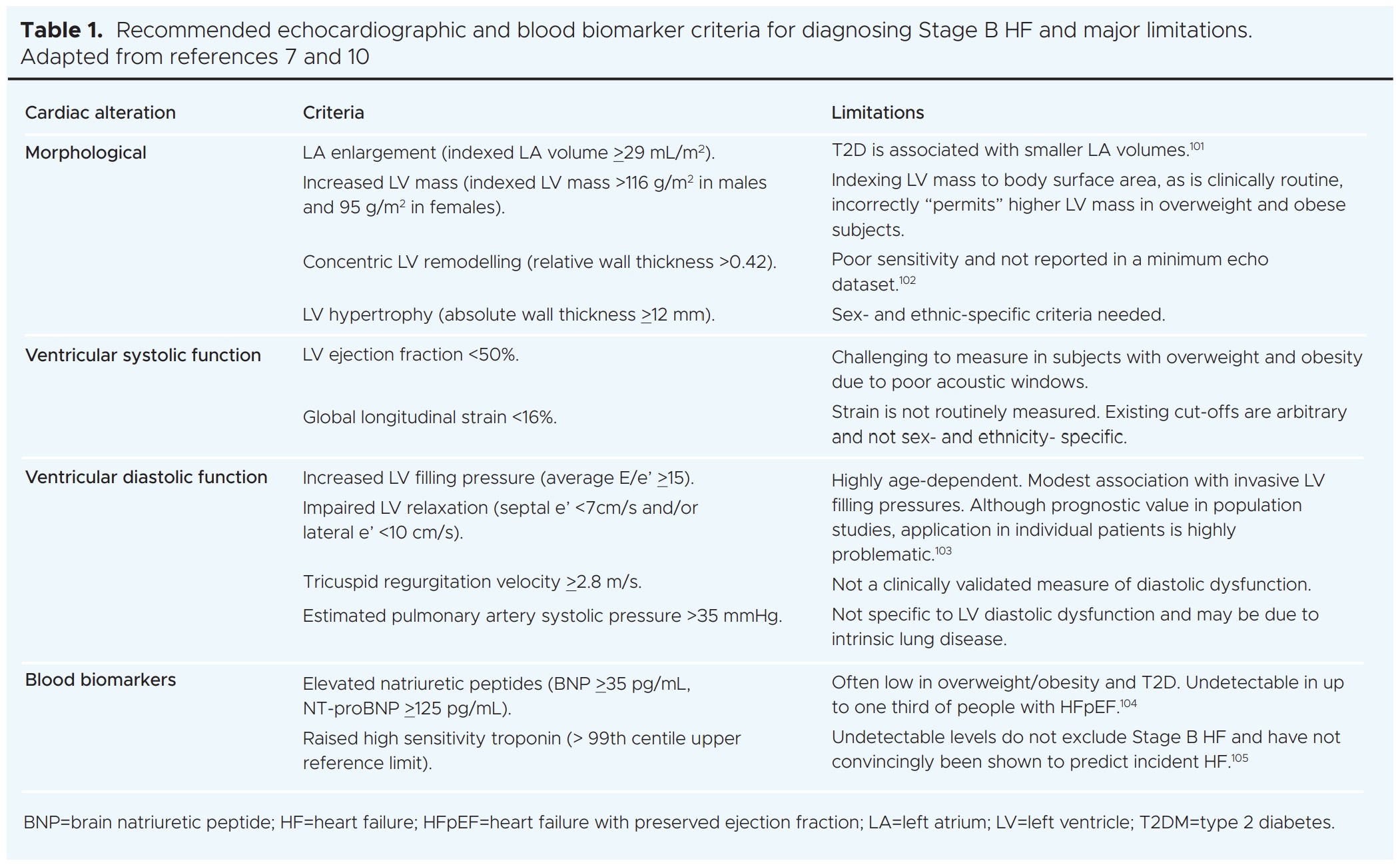
Surprisingly, very few available risk stratification tools for CV disease in T2DM are calibrated for HF risk estimation.98 There is a paucity of generalisable or robust T2DM-specific HF risk calculators, such that risk stratification is highly challenging in real-world practice.99 For this reason, emphasis should be on early initiation and maintenance of the cardio-protective glucose-lowering agents.
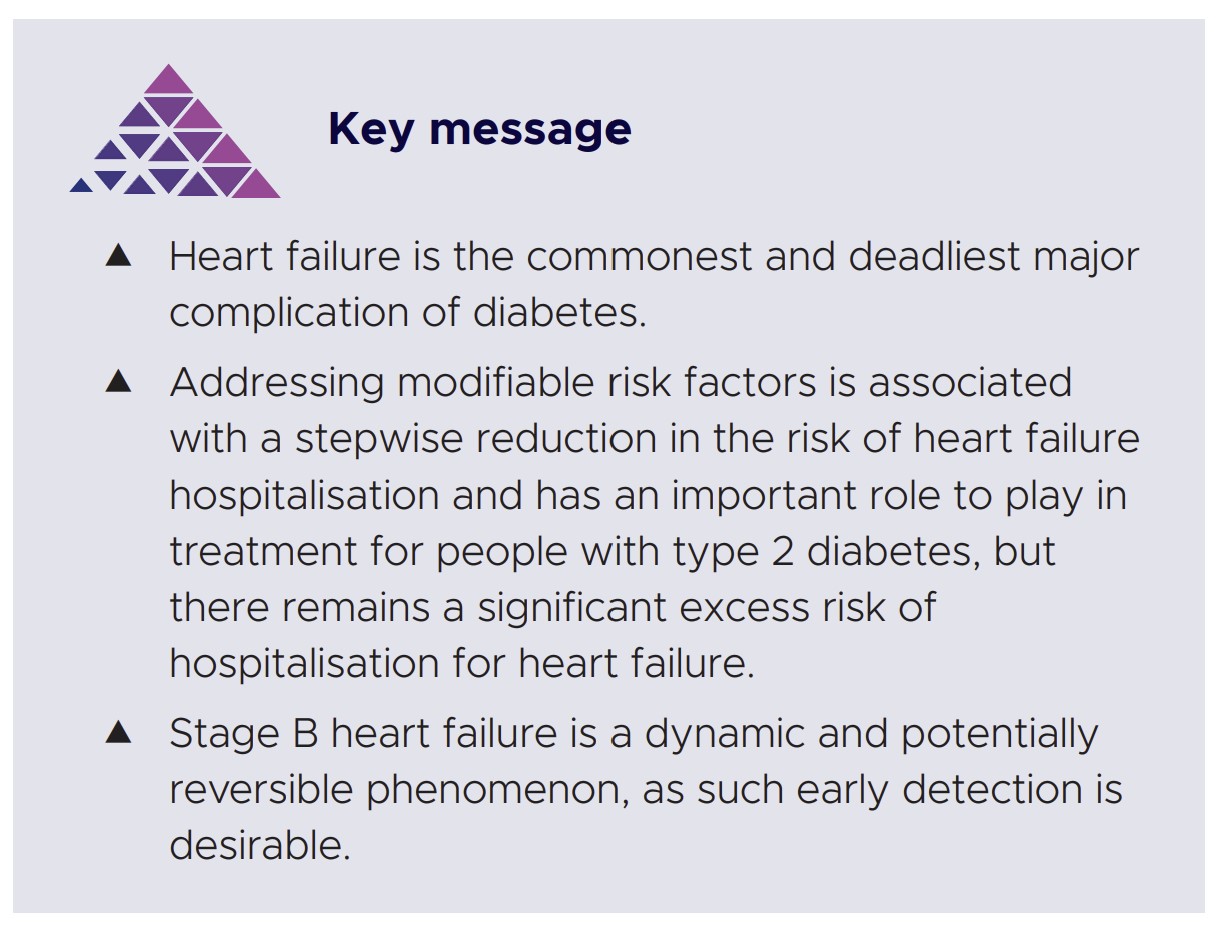
Management of Stage B HF follows the same principles as for Stage A. Several studies have shown that lifestyle modification and risk factor control can ameliorate many of the cardiac alterations described in Stage B HF (Table 2). Where evidence of a reduced LV ejection fraction is opportunistically detected, early initiation of guideline-directed medical therapy is strongly encouraged. Conversely, avoidance of thiazolidinediones, dipeptidyl peptidase IV inhibitors and sulfonylureas is advised, given the association with risk of CV events and HF.6,100 The absolute goal is to prevent deterioration of cardiac dysfunction and progression to symptomatic HF.
Conclusions
Often underappreciated as a complication of T2DM, HF is one of the commonest and deadliest consequences of the disease. All the available evidence suggests that in people with T2DM and Stage A HF: 1) intensive blood glucose control is not sufficient for HF prevention and a multifaceted approach is likely to have a greater effect; 2) the most compelling evidence for HF risk reduction is for SGLT2i, although GLP-1 RA may also have a role; and 3) patients likely to derive most benefit are those at highest risk of developing overt HF, which probably represents the majority of people with T2DM. Increased awareness and early diagnosis are paramount to establish treatment, and more research is required to identify patients with stage B HF effectively in this at-risk cohort.

© 2024. This work is openly licensed via CC BY 4.0.
This license enables reusers to distribute, remix, adapt, and build upon the material in any medium or format, so long as attribution is given to the creator. The license allows for commercial use. CC BY includes the following elements: BY – credit must be given to the creator.
Copyright ownership The author(s) retain copyright.
Conflict of interest None.
Funding AC, EMB, and GPM received funding from the National Institute for Health and Care Research (NIHR) United Kingdom through a Research Professorship award (RP-2017-08-ST2-007). AD received funding from the British Heart Foundation through a Clinical Research Training Fellowship (FS/CRTF/20/24069). GSG is funded by the NIHR, through a Clinical Lectureship.
Acknowledgements Figure created using biorender.com.
References
-
Rawshani A, Rawshani A, Franzén S, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 NEnglJ Med 2018;379(7):633-44. https://doi.org/10.1056/NEJMoa1800256
-
Health and Social Care Information Are diabetes services in England and Wales Measuring up? A summary of findings from the National Diabetes Audit 2011–12 for people with diabetes and anyone interested in the quality of diabetes care National Diabetes Audit 2011-12. 2012. hscic.gov.uk/catalogue/PUB12421
-
(HQIP) THQIP. National Diabetes Audit, 2017-18. Report 2a: Complications and Mortality (complications of diabetes) England and Wales. Healthcare Quality Improvement Partnership. 2017-18. www.hqip.org.uk
-
(HQIP) THQIP. National Diabetes Audit, 2017-18 Report 2b: Complications and Mortality (characteristics associated with adverse cardiovascular outcomes and diabetic complications) England and 2019. www.hqip.org.uk
-
Centre HaSCI. National Diabetes Audit 2010-2011 Report 2: Complications and Mortality. 2012. www.digital.nhs.uk
-
Pop-Busui R, Januzzi JL, Bruemmer D, et al. Heart Failure: an underappreciated complication of A consensus report of the American Diabetes Association. Diabetes Care 2022;45(7):1670-90. https://doi.org/10.2337/dci-0014
-
Dolgin M, Committee NYHA Criteria Commitee. Nomenclature and criteria for diagnosis of diseases of the heart and great vessels: Little, Brown; Boston, 1994.
-
McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart Eur Heart J 2021;42(36):3599-726. https://doi.org/10.1093/eurheartj/ehab368
-
Heidenreich PA, Bozkurt B, Aguilar D, et 2022 AHA/ACC/HFSA Guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation 2022; 145(18):e895-e1032. https://doi.org/10.1161/CIR.00000000001063
-
Hunt SA, Baker DW, Chin MH, et ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary. A report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee to revise the 1995 guidelines for the evaluation and management of heart failure). Circulation 2001;104(24):2996- 3007. https://doi.org/10.1161/hc4901.102568
-
Bozkurt B, Coats A, Tsutsui Universal definition and classification of heart failure. J Card Fail 2021;23:352-80. https://doi.org/10.1002/ejhf.2115
-
Ammar KA, Jacobsen SJ, Mahoney DW, et al. Prevalence and prognostic significance of heart failure Circulation 2007; 115(12):1563-70. https://doi.org/10.1161/CIRCULATIONAHA.106.666818
-
ACCORDION: long-term follow-up of ACCORD patients. American Heart Association (AHA) 2015 Scientific Sessions; 2015; Orlando,
-
Castagno D, Baird-Gunning J, Jhund PS, et Intensive glycemic control has no impact on the risk of heart failure in type 2 diabetic patients: evidence from a 37,229 patient meta-analysis. Am Heart J 2011;162(5):938-48.e2. https://doi.org/10.j.ahj.2011.07.030
-
ACCORD study Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362(17):1575-85. https://doi.org/10.1056/NEJMoa1001286
-
Kamimura D, Cain LR, Mentz RJ, etal. Cigarette smoking and incident heart Circulation 2018;137(24):2572-82. https://doi.org/10.1161/CIRCULATIONAHA.117.031912
-
Watson M, Dardari Z, Kianoush S, etal. Relation between cigarette smoking and heart failure (from the Multiethnic Study of Atherosclerosis). Am J Cardiol 2019;123(12):1972-7. https://doi.org/1016/j.amjcard.209.03.015
-
Gopal DM, Kalogeropoulos AP, Georgiopoulou VV, et al. Cigarette smoking exposure and heart failure risk in older adults: The Health, Aging, and Body Composition Study. Am Heart J 2012;164(2):236- https://doi.org/10.1016/j.ahj.2012.05.013
-
Ahmed AA, Patel K, Nyaku MA, et Risk of heart failure and death after prolonged smoking cessation. Circulation Heart Fail 2015; 8(4):694-701. https://doi.org/10.1161/CIRCHEARTFAILURE.114.001885
-
Kennedy CD, Van Schalkwyk MCI, McKee M, Pisinger C. The cardiovascular effects of electronic cigarettes: a systematic review of experimental studies. Preventive Medicine 2019;127:105770. https://doi.org/10.1016/j.ypmed.2019.105770
-
Study links e-cigarette use with higher risk of heart Large study adds to growing body of evidence that vaping may harm the heart [press release]. American College of Cardiology, 2024.
-
Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med 2000; 132(8):605-11. https://doi.org/10.7326/0003-4819-132-8-20004180-00002
-
Hu FB, Stampfer MJ, Solomon C, et al. Physical activity and risk for cardiovascular events in diabetic Ann Intern Med 2001; 134(2):96-105. https://doi.org/10.7326/0003-4819-134-2-200101160-00009
-
He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up ArchInternMed 2001;161(7):996- 1002. https://doi.org/10.1001/archinte.161.5.996
-
Kenchaiah S, Sesso HD, Gaziano Body mass index and vigorous physical activity and the risk of heart failure among men. Circulation 2009;119(1):44-52. https://doi.org/10.1161/CIRCULATIONAHA.108.807289
-
Hu G, Jousilahti P, Antikainen R, Katzmarzyk PT, Tuomilehto Joint effects of physical activity, body mass index, waist circumference, and waist-to-hip ratio on the risk of heart failure. Circulation 2010; 121(2):237-44. https://doi.org/10.1161/CIRCULATIONAHA.109.887893
-
Kraigher‐Krainer E, Lyass A, Massaro JM, et Association of physical activity and heart failure with preserved vs. reduced ejection fraction in the elderly: the Framingham Heart Study. Eur J Heart Fail 2013; 15(7):742-6. https://doi.org/10.1093/eurjhf/hft025
-
Pandey A, Patel M, Gao A, et Changes in mid-life fitness predicts heart failure risk at a later age independent of interval development of cardiac and noncardiac risk factors: The Cooper Center Longitudinal Study. Am Heart J 2015;169(2):290-7.e1. https://doi.org/10.1016/j.ahf.2014.10.017
-
Khan H, Kunutsor S, Rauramaa R, et Cardiorespiratory fitness and risk of heart failure: a population‐based follow‐up study. Eur J Heart Fail 2014;16(2):180-8. https://doi.org/10.1111/ejhf.37
-
Florido R, Kwak L, Lazo M, et al. Physical activity and incident heart failure in high‐risk subgroups: the ARIC JournalAmerican Heart Association 2020;9(10):e014885 https://doi.org/10.1161/JAHA.119.014885
-
Umpierre D, Ribeiro PA, Kramer CK, etal. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA 2011; 305(17):1790-9. https://doi.org/10.1001/jama.2011.576
-
Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical JAMA 2001; 286(10):1218-27. https://doi.org/10.1001/jama.286.10.1218
-
Colberg SR, Sigal RJ, Yardley JE, et Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care 2016;39(11):2065-79. https://doi.org/10.2337/dc16-1728
-
Marx N, Federici M, Schütt K, et al, and ESC Scientific Document 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J 2023;44:4043-140.. https://doi.org/10.1093/eurheartj/ehad192
-
Kemps H, Krankel N, Dorr M, et Exercise training for patients with type 2 diabetes and cardiovascular disease: what to pursue and how to do it. A position paper of the European Association of Preventive Cardiology (EAPC). Eur J Prev Cardiol 2019;26:709-27. https://doi.org/10.1177/2047487318820420.
-
Lee DS, Massaro JM, Wang TJ, etal. Antecedent blood pressure, body mass index, and the risk of incident heart failure in later Hypertension 2007;50(5):869-76. https://doi.org/10.1161/HYPERTENSIONAHA.107.095380
-
Li W, Katzmarzyk PT, Horswell R, et al. Body mass index and heart failure among patients with type 2 diabetes mellitus. Circulation Heart Failure 2015;8(3):455-63. https://doi.org/10.1161/CIRCHEARTFAILURE.114.001837
-
Wilding JPH. The importance of weight management in type 2 diabetes Int J Clin Pract 2014;68(6):682-91. https://doi.org/10.1111/ijcp.12384
-
Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 Diabetes Care 2011;34(7):1481-6. https://doi.org/10.2337/dc10-2415
-
Williamson DF, Thompson TJ, Thun M, Flanders D, Pamuk E, Byers Intentional weight loss and mortality among overweight individuals with diabetes. Diabetes Care 2000;23(10):1499-504. https://doi.org/10.2337/diacare.23.10.1499
-
Pandey A, Patel KV, Bahnson JL, et al. Association of intensive lifestyle intervention, fitness, and body mass index with risk of heart failure in overweight or obese adults with type 2 diabetes Circulation 2020;141(16):1295-306. https://doi.org/10.1161/CIRCULATIONAHA.119.044865
-
Look ARG, Wing RR, Bolin P, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013; 369(2):145-54. https://doi.org/10.1056/NEJMoa1212914
-
Look ARG, Gregg EW, Jakicic JM, etal. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol 2016;4(11):913-21. https://doi.org/10.1016/S2213-8586(16)30162-0
-
Patel KV, Bahnson JL, Gaussoin SA, et Association of baseline and longitudinal changes in body composition measures with risk of heart failure and myocardial infarction in type 2 diabetes: findings from the Look AHEAD Trial. Circulation 2020;142(25):2420-30. https://doi.org/10.1161/CIRCULATIONAHA.120.050941
-
Diallo A, Villard O, Carlos-Bolumbu M, Renard E, Galtier Effects of hypoglycaemic agents on reducing surrogate metabolic parameters for the prevention of cardiovascular events and death in patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab 2023;26:495-502. https://doi.org/10.1111/dom.15335
-
Jastreboff AM, Aronne LJ, Ahmad NN, etal. Tirzepatide once weekly for the treatment of obesity. N Engl J Med 2022;387(3):205-16. https://doi.org/10.1056/NEJMoa2206038
-
Garvey WT, Frias JP, Jastreboff AM, et Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo- controlled, phase 3 trial. Lancet 2023;402(10402):613-26. https://doi.org/10.1016/S0140-6736(23)01200-X
-
Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or N Engl J Med 2021; 384(11):989. https://doi.org/10.1056/NEJMoa2032183
-
Pi-Sunyer X, Astrup A, Fujioka K, et A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 2015; 373(1):11-22. https://doi.org/10.1056/NEJMoa1411892
-
Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 N Engl J Med 2016; 375(4):311-22. https://doi.org/10.1056/NEJMoa1603827
-
Marso SP, Bain SC, Consoli A, et Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375(19):1834-44. https://doi.org/10.1056/NEJMoa1607141
-
Lincoff AM, Brown-Frandsen K, Colhoun HM, et al. Semaglutide and cardiovascular outcomes in obesity without N Engl J Med 2023;389:2221-32. https://doi.org/10.1056/NEJMoa2307563
-
Lee MMY, Sattar N, McMurray JJV, Packard CJ. Statins in the prevention and treatment of heart failure: a review of the Current Atherosclerosis Reports 2019;21(10):41. https://doi.org/10.1007/s11883-019-0800-z
-
Lakoumentas JA, Dimitroula TG, Aggeli KI, Harbis Cholesterol levels and the benefit of statins in heart failure. Hellenic J Cardiol 2005;46(3):226-31.
-
Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled Lancet 2004;364(9435):685-96. https://doi.org/10.1016/S0140-6736(04)16895-5
-
SSSS Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383-9. https://doi.org/1016/S0140-6736(94)90566-5
-
Low Wang CC, Hess CN, Hiatt WR, Goldfine Clinical update: cardiovascular disease in diabetes mellitus. Circulation 2016;133 (24):2459-502. https://doi.org/10.1161/CIRCULATIONAHA.116.022194
-
Filippatos TD, Mikhailidis Statins and heart failure. Angiology 2008;59(2 Suppl):58s-61s. https://doi.org/10.1177/0003319708319643
-
Bielecka-Dabrowa A, Bytyçi I, Von Haehling S, et al. Association of statin use and clinical outcomes in heart failure patients: a systematic review and meta-analysis. Lipids in Health and Disease 2019;18(1):188. https://doi.org/10.1186/s12944-019-1135-z
-
Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary N Engl J Med 2018;379(22):2097-107. https://doi.org/10.1056/NEJMoa1801174
-
JBS3 Summary of national guidance for lipid management for primary and secondary prevention of CVD. In: Collaborative NHSAA, editor. 2024.
-
Niessner A, Drexel PCSK9 inhibition in patients with heart failure: neutral or harmful intervention? Eur Heart J 2022;43(16):1566-8. https://doi.org/10.1093/eurheartj/ehab913
-
Da Dalt L, Castiglioni L, Baragetti A, etal. PCSK9 deficiency rewires heart metabolism and drives heart failure with preserved ejection Eur Heart J 2021;42(32):3078-90. https://doi.org/10.1093/eurheartj/ehab431
-
Cegla National Institute for Health and Care Excellence guidelines for lipid management. Heart 2023;109(9):661-7. https://doi.org/10.1136/heartjnl-2022-321414
-
Echouffo-Tcheugui JB, Ndumele CE, Zhang S, et Diabetes and progression of heart failure: the Atherosclerosis Risk In Communities (ARIC) Study. J Am Coll Cardiol 2022;79(23):2285-93. https://doi.org/10.1016/j.jacc.2022.03.378
-
Lind M, Olsson M, Rosengren A, Svensson AM, Bounias I, Gudbjörnsdottir The relationship between glycaemic control and heart failure in 83,021 patients with type 2 diabetes. Diabetologia 2012;55(11):2946-53. https://doi.org/10.1007/s00125-012-2681-3
-
ADVANCE Collaborative Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352(9131):854-65.
-
Group AC, Patel A, MacMahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 N Engl J Med 2008;358(24):2560-72. https://doi.org/10.1056/NEJMoa0802987
-
Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 N Engl J Med 2008;358(24):2545-59. https://doi.org/10.1056/NEJMoa0802743
-
Duckworth W, Abraira C, Moritz T, et Glucose control and vascular complications in veterans with type 2 diabetes. NEnglJMed 2009; 360(2):129-39. https://doi.org/10.1056/NEJMoa0808431
-
Control Group; Turnbull FM, Abraira C, Anderson RJ, et Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 2009;52(11):2288-98. https://doi.org/10.1007/s00125-009-1470-0
-
ElSayed NA, Aleppo G, Aroda VR, et al. Cardiovascular disease and risk management: standards of care in diabetes-2023. Diabetes Care 2023;46(Suppl 1):S158-S90. https://doi.org/10.2337/dc23-S010
-
Cosentino F, Grant PJ, Aboyans V, et 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2020;41(2):255-323. https://doi.org/10.1093/eurheartj/ehz486
-
Type 2 diabetes in adults: management. 2015. PMID:26741015
-
Joseph JJ, Deedwania P, Acharya T, et al. Comprehensive management of cardiovascular risk factors for adults with type 2 diabetes: a scientific statement from the American Heart Association. Circulation 2022;145(9): e722-e759. https://doi.org/10.1161/CIR00000000001040
-
Zinman B, Wanner C, Lachin JM, etal. Empagliflozin, cardiovascular outcomes, and mortality in type 2 N Engl J Med 2015;373(22):2117-28. https://doi.org/10.1056/NEJMoa1504720
-
Wiviott SD, Raz I, Bonaca MP, et Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347-57. https://doi.org/10.1056/NEJMoa1812389
-
Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 N Engl J Med 2017;377(7):644-57. https://doi.org/10.1056/NEJMoa1611925
-
McGuire DK, Shih WJ, Cosentino F, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol 2021;6(2):148-58. https://doi.org/10.1001/jamacardio.2020.4511
-
Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 N Engl J Med 2017;377(13):1228-39. https://doi.org/10.1056/NEJMoa1612917
-
Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 2018;392:1519-29. https://doi.org/10.1016/S0140-6736(18)32261-X
-
Husain M, Birkenfeld AL, Donsmark M, et Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. NEnglJ Med 2019;381:841-51. https://doi.org/10.1056/NEJMoa1901118
-
Ferreira JP, Saraiva F, Sharma A, et al. Glucagon-like peptide 1 receptor agonists in patients with type 2 diabetes with and without chronic heart failure: a meta-analysis of randomized placebo- controlled outcome trials. Diabetes Obes Metab 2023;25(6): 1495-502. https://doi.org/10.1111/dom.14997
-
Bayés-Genís A, Díez Transition to heart failure in hypertension: going to the heart of the matter. EurHeartJ 2022;43(35):3332-4. https://doi.org/10.1093/eurheartj/ehab651
-
Goyal A, Norton CR, Thomas TN, et al. Predictors of incident heart failure in a large insured population: a one million person-year follow- up study. Circ Heart Fail 2010;3(6):698-705. https://doi.org/10.1161/110.938175
-
Ahmad FS, Ning H, Rich JD, Yancy CW, Lloyd-Jones DM, Wilkins Hypertension, obesity, diabetes, and heart failure-free survival: the Cardiovascular Disease Lifetime Risk Pooling Project. JACC Heart Fail 2016;4(12):911-19. https://doi.org/10.1016/j.jchf.2016.08.001
-
Folsom AR, Yamagishi K, Hozawa A, Chambless Absolute and attributable risks of heart failure incidence in relation to optimal risk factors. Circulation Heart Failure 2009;2(1):11-17. https://doi.org/10.1161/CIRCHEARTFAILURE.108.794933
-
The SPRINT Research A randomized trial of intensive versus standard blood-pressure control. N EngJ Med 2015;373(22):2103-16. https://doi.org/10.1056/NEJMoa1511939
-
Hypertension in adults: diagnosis and management. 2019. NG136
-
Weinstock RS, Trief PM, Burke B, etal. Antihypertensive and lipid- lowering medication adherence in young adults with youth-onset type 2 diabetes. JAMA Network Open 2023;6(10):e2336964. https://doi.org/10.1001/jamanetworkopen.2023.36964
-
Gupta P, Patel P, Štrauch B, et al. Risk factors for nonadherence to antihypertensive Hypertension 2017;69(6):1113-20. https://doi.org/10.1161/HYPERTENSIONAHA.116.08729
-
Gupta P, Patel P, Horne R, Buchanan H, Williams B, Tomaszewski How to screen for non-adherence to antihypertensive therapy. Current Hypertension Reports 2016;18(12):89. https://doi.org/10.1007/s11906-016-0697-7
-
Boonman-de Winter LJ, Rutten FH, Cramer MJ, et High prevalence of previously unknown heart failure and left ventricular dysfunction in patients with type 2 diabetes. Diabetologia 2012;55(8):2154-62. https://doi.org/10.1007/s00125-012-2579-0
-
Echouffo-Tcheugui JB, Erqou S, Butler J, Yancy CW, Fonarow Assessing the risk of progression from asymptomatic left ventricular dysfunction to overt heart failure: a systematic overview and meta- analysis. JACC Heart Fail 2016;4(4):237-48. https://doi.org/10.1016/j.jchf.2015.09.015
-
Young KA, Scott CG, Rodeheffer RJ, Chen HH. Progression of preclinical heart failure: a description of stage A and B heart failure in a community population. Circ Cardiovasc Qual Outcomes 2021; 14(5):e007216. https://doi.org/10.1161/CIRCOUTCOMES.120.007216
-
Gulsin GS, Athithan L, McCann GP. Diabetic cardiomyopathy: prevalence, determinants and potential treatments. Ther Adv Endocrinol Metab 2019;10:2042018819834869. https://doi.org/10.1177/204201881983489
-
Dattani A, Joshi S, Yeo JL, et Impaired myocardial calcium uptake in patients with diabetes mellitus: a manganese-enhanced cardiac magnetic resonance study. JACC Cardiovasc Imaging 2023; 16(12):1623-5. https://doi.org/10.1016/j.jcmg.202.05.009
-
SCORE2-Diabetes Working Group and the ESCCRC. SCORE2- Diabetes: 10-year cardiovascular risk estimation in type 2 diabetes in Eur Heart J 2023;44(28):2544-56. https://doi.org/10.1097/eurheartj/ehad260
-
Pandey A, Khan MS, Patel KV, Bhatt DL, Verma S. Predicting and preventing heart failure in type 2 diabetes. Lancet Diabetes Endocrinol 2023;11(8):607-24. https://doi.org/10.1016/S2213-8587(23)00128-6
-
Azimova K, San Juan Z, Mukherjee D. Cardiovascular safety profile of currently available diabetic OchsnerJ 2014;14(4):616-32. PMID:25598727
-
Jensen MT, Fung K, Aung N, etal. Changes in cardiac morphology and function in individuals with diabetes mellitus: the UK Biobank cardiovascular magnetic resonance substudy. Circ Cardiovasc Imaging 2019;12(9):e009476. https://doi.org/10.1161/119.009476
-
Robinson S, Rana B, Oxborough D, et A practical guideline for performing a comprehensive transthoracic echocardiogram in adults: the British Society of Echocardiography minimum dataset. Echo Res Pract 2020;7(4):G59-G93. https://doi.org/10.1530/ERP-20-0026
-
Mitter SS, Shah SJ, Thomas A test in context: E/A and E/e' to assess diastolic dysfunction and LV filling pressure. J Am Coll Cardiol 2017;69(11):1451-64. https://doi.org/10.1016/j.jacc.2016.12.037
-
Verbrugge FH, Omote K, Reddy YNV, Sorimachi H, Obokata M, Borlaug Heart failure with preserved ejection fraction in patients with normal natriuretic peptide levels is associated with increased morbidity and mortality. Eur Heart J 2022;43(20):1941-51. https://doi.org/10.1093/eurheartj/ehab911
-
Ohkuma T, Jun M, Woodward M, et al. Cardiac stress and inflammatory markers as predictors of heart failure in patients with type 2 diabetes: the ADVANCE Diabetes Care 2017;40(9):1203-9. https://doi.org/10.2337/dc17-0509
-
Bojer AS, Sørensen MH, Gæde P, Madsen PL. Myocardial extracellular volume expansion in type 2 diabetes is associated with ischemic heart disease, autonomic neuropathy, and active Diabetes Care 2022;45(12):3032-9. https://doi.org/10.2337/dc22-0942
-
Gulsin GS, Swarbrick DJ, Athithan L, etal. Effects of low-energy diet or exercise on cardiovascular function in working-age adults with type 2 diabetes: a prospective, randomized, open-label, blinded end point trial. Diabetes Care 2020;43(6):1300-10. https://doi.org/2337/dc20-0129
-
Liakopoulos V, Franzén S, Svensson AM, et al. Renal and cardiovascular outcomes after weight loss from gastric bypass surgery in type 2 diabetes: cardiorenal risk reductions exceed atherosclerotic benefits. Diabetes Care 2020;43(6):1276-84. https://doi.org/10.2337/dc19-1703
-
Skali H, Shah A, Gupta DK, et al. Cardiac structure and function across the glycemic spectrum in elderly men and women free of prevalent heart disease: the Atherosclerosis Risk In the Community Circ Heart Fail 2015;8(3):448-54. https://doi.org/10.1161/CIRCHEARTFAILURE.114.001990.